Lab of Sensory & Emotion Neuroscience
Hyeonwi Son, Ph.D.
Research Interests
At the SENS Lab (Sensory & Emotion Neuroscience Laboratory), we focus on understanding how sensory and emotional processes are shaped by the peripheral nervous system and how these mechanisms contribute to pain and psychiatric disorders. Our research integrates advanced in vivo imaging, AI-driven behavioral analysis, and molecular approaches to bridge the gap between peripheral and central nervous system function.
Our main research interests include:
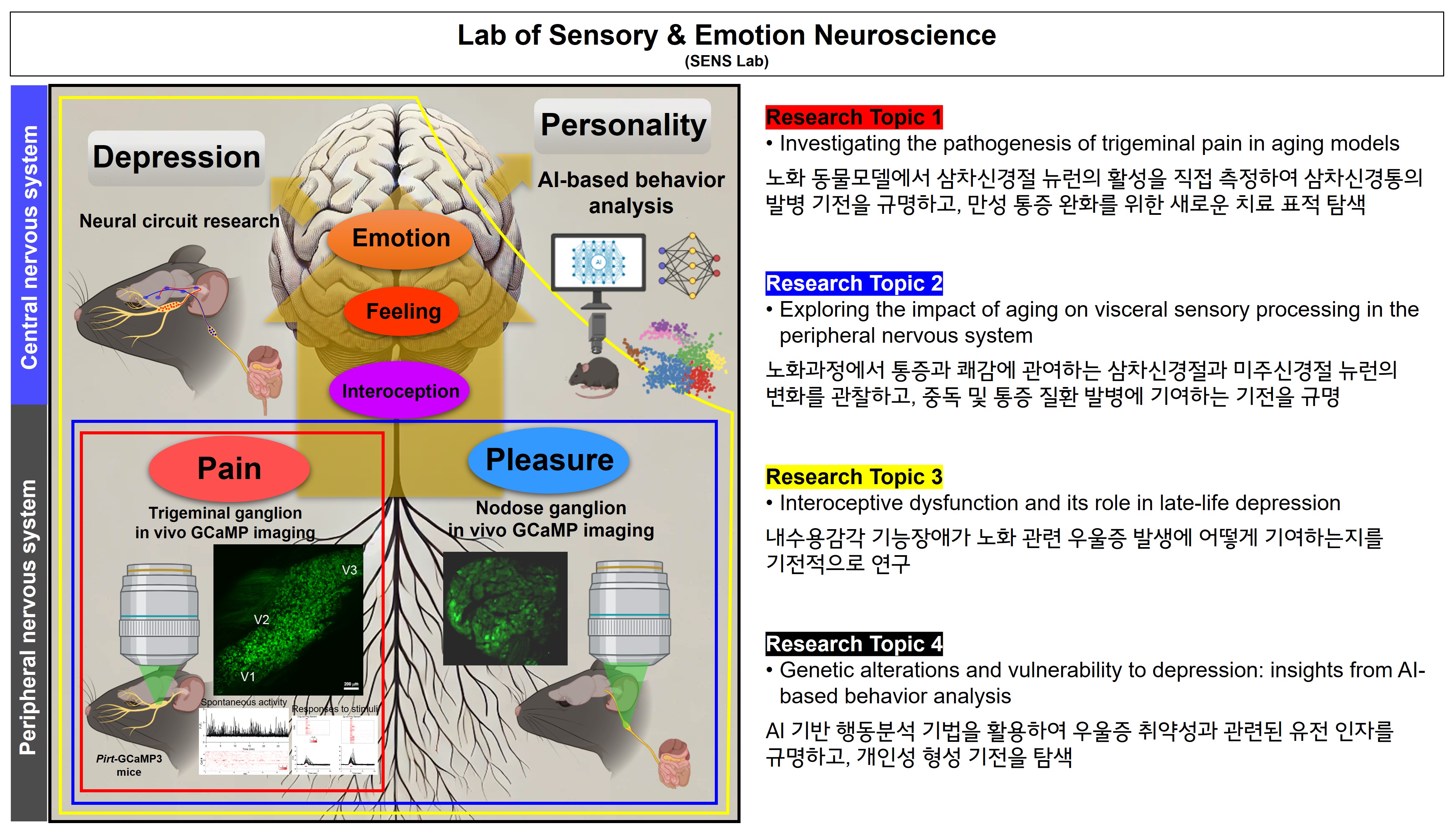
• Mechanisms of trigeminal pain in aging: Investigating how age-related changes in trigeminal ganglion neurons drive the development of trigeminal neuralgia and chronic pain, with the goal of identifying novel therapeutic targets.
• Aging and visceral sensory processing: Exploring how aging alters sensory signaling in the trigeminal and nodose ganglia, and how these changes contribute to addiction and pain-related disorders.
• Interoception and late-life depression: Examining how dysfunction in interoceptive processing influences emotional regulation and predisposes individuals to depression during aging.
• Genetic alterations and vulnerability to depression: Using AI-based behavioral analysis to uncover genetic factors that underline depression susceptibility and shape personality traits.
Through these studies, we aim to uncover fundamental mechanisms linking the peripheral nervous system to emotion, cognition, and behavior, ultimately contributing to the development of new strategies for treating chronic pain and neuropsychiatric disorders.
Selected Publications
1. Alshanqiti, I.*, Son, H.*, Shannonhouse, J., Hu, J., Kumari, S., Parastooei, G., Raman, S., Wang, S., Ro, J.Y., Kim, Y.S., et al. (2024). Posttraumatic hyperalgesia and associated peripheral sensitization after temporomandibular joint injury in mice. Pain 166, 1597–1609. 10.1097/j.pain.0000000000003498. (*Equal contributions as co-first authors)
2. Son, H., Shannonhouse, J., Zhang, Y., Gomez, R., Amarista, F., Perez, D., Ellis, E., Chung, M.-K., and Kim, Y.S. (2024). Elucidation of neuronal activity in mouse models of temporomandibular joint injury and inflammation by in vivo GCaMP Ca 2+ imaging of intact trigeminal ganglion neurons. Pain 165, 2794–2803. 10.1097/j.pain.0000000000003421.
3. Son, H., Zhang, Y., Shannonhouse, J., Gomez, R., and Kim, Y.S. (2024). PACAP38/mast-cell-specific receptor axis mediates repetitive stress-induced headache in mice. J. Headache Pain 25, 87. 10.1186/s10194-024-01786-3.
4. Son, H., Zhang, Y., Shannonhouse, J., Ishida, H., Gomez, R., and Kim, Y.S. (2024). Mast-cell-specific receptor mediates alcohol-withdrawal-associated headache in male mice. Neuron 112, 113-123.e4. 10.1016/j.neuron.2023.09.039.
5. Baek, J.H.*, Son, H.*, Kang, J.S., Yoo, D.Y., Chung, H.J., Lee, D.K., and Kim, H.J. (2022). Long-Term Hyperglycemia Causes Depressive Behaviors in Mice with Hypoactive Glutamatergic Activity in the Medial Prefrontal Cortex, Which Is Not Reversed by Insulin Treatment. Cells 11. (*Equal contributions as co-first authors)
6. Son, H., Baek, J.H., Kang, J.S., Jung, S., Chung, H.J., and Kim, H.J. (2021). Acutely increased β-hydroxybutyrate plays a role in the prefrontal cortex to escape stressful conditions during the acute stress response. Biochem. Biophys. Res. Commun. 554, 19–24.
7. Son, H.*, Kim, S.*, Jung, D.-H., Baek, J.H., Lee, D.H., Roh, G.S., Kang, S.S., Cho, G.J., Choi, W.S., Lee, D.K., et al. (2019b). Insufficient glutamine synthetase activity during synaptogenesis causes spatial memory impairment in adult mice. Sci. Rep. 9. (*Equal contributions as co-first authors)
8. Son, H., Jung, S., Shin, J., Kang, M., and Kim, H. (2018a). Anti-Stress and Anti-Depressive Effects of Spinach Extracts on a Chronic Stress-Induced Depression Mouse Model through Lowering Blood Corticosterone and Increasing Brain Glutamate and Glutamine Levels. J. Clin. Med. 7, 406.
9. Son, H., Baek, J.H., Go, B.S., Jung, D., Sontakke, S.B., Chung, H.J., Lee, D.H., Roh, G.S., Kang, S.S., Cho, G.J., et al. (2018b). Glutamine has antidepressive effects through increments of glutamate and glutamine levels and glutamatergic activity in the medial prefrontal cortex. Neuropharmacology.
10. Son, H., Jung, S., Kim, J.Y., Goo, Y.M., Cho, K.M., Lee, D.H., Roh, G.S., Kang, S.S., Cho, G.J., Choi, W.S., et al. (2015). Type 1 diabetes alters astrocytic properties related with neurotransmitter supply, causing abnormal neuronal activities. Brain Res 1602, 32–43.
11. Lee, Y., Son, H., Kim, G., Kim, S., Lee, D.H., Roh, G.S., Kang, S.S., Cho, G.J., Choi, W.S., and Kim, H.J. (2013). Glutamine deficiency in the prefrontal cortex increases depressive-like behaviours in male mice. J. Psychiatry Neurosci. 38.


